Ellipsometry
Techniques: Ellipsometry
Ellipsometry is a well-established technique for determining the optical properties of thin films and surfaces. It is used extensively in my group to quantify dynamic surfactant adsorption at solid�liquid, liquid�liquid and liquid�air interfaces, and to investigate interfacial phase transitions. It has sub-second kinetic resolution, is sensitive to a sub-monolayer surface coverage down to ~1% of a monolayer and is also relatively simple to implement; merely requiring that the surface can reflect a laser beam. A picture of the ellipsometer is shown below.
The electric vector of a laser beam reflecting at an interface may be split up into two perpendicular components: one in the plane of incidence called p-polarised light and the other in the plane of the interface called s-polarised light. The proportion of light reflected an interface depends on the polarisation of light and the angle of incidence. Reflection from an interface alters the polarisation of light in a well-defined way and ellipsometry measures the ratio of the reflection coefficients of p and s-polarised light from an interface. At the Brewster angle in the thin film limit (film thickness much less than the incident wavelength), the real part of this ratio is zero and the imaginary part is known as the coefficient of ellipticity. The ellipticity is extremely sensitive to perturbations in the dielectric constant profile across the interface caused by thermal roughening and the presence of a thin film.
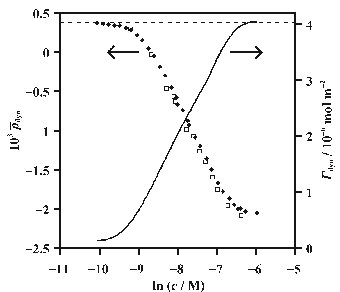
Ellipsometry is an under-determined technique, i.e. it yields one parameter (the ellipticity) that is dependent on two variables (the dielectric constant and thickness of the interfacial layer). This problem can often be circumvented to give information inaccessible to other techniques, e.g. by calibration with values of the surface tension measured with TENSIOMETRY or values of the surface excess measured with NEUTRON REFLECTION. In my group we have ascertained that the coefficient of ellipticity is proportional to the surface excess for certain surfactants. This linearity enables quantification of the dynamic adsorption for systems such as the liquid jet, overflowing cylinder (OFC) and the channel flow cell. In the figure above the ellipticity and surface excess of the cationic surfactant hexadecyltrimethylammonium bromide (C16TAB) in the OFC are plotted as a function of concentration. The surface excess is calibrated against the ellipticity to enable rapid determination of dynamic surface excess from ellipsometry measurements.
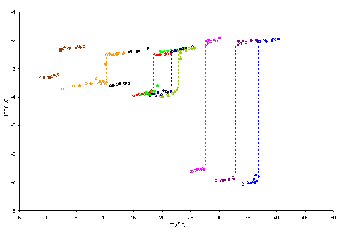
Surface phase transitions such as surface freezing produce large changes in the coefficient of ellipticity. In the figure above, the coefficient of ellipticity is plotted as a function of temperature for a series of mixed monolayers of C16TAB and simple alkanes (undecane to eicosane) at the air�water interface. The discontinuities are indicative of a phase transition and reasonable assumptions in the model can lead to the acquisition of compositional and structural information about the monolayer close to the phase transition.
